Who are we ?
Created by Serge Calamel in Marseille in 1984, Biotechni is a french manufacturer of orthopedic implants, specialized in hip, shoulder and spine surgery.
Biotechni is recognized as an innovative, reliable french player and engaged in orthopedic surgery.
Holder of several patents and brands, Biotechni continues to develop and improve its products in collaboration with french and international medical teams.
In this constantly evolving and very competitive field, Biotechni has managed to keep its independence in order to be able to make its own choices.

French Manufacturer of orthopedics implants since 1984

Ranked among the top 50 European orthopedic implant manufacturers by Research facts LTD in 2012
Know-how
Biotechni has been capitalizing for 36 years in the development and manufacture of orthopedic implants.
To do this, Biotechni has continuously invested in state-of-the-art equipment and human resources.
With constantly evolving regulations, Biotechni has always been able to adapt to very strict requirements and obtain certifications for its products.
Listed in several arthroplasty registries and supported by its scientific studies for more than 20 years, Biotechni has a success rate that demonstrates the excellence of the quality and reliability of its products.
Our know-how is the result of close collaboration with surgical and scientific teams.

Quality
Certifications and regulatory
Biotechni est certifiée ISO 13485

French Manufacturer of orthopeadics implants since 1984

Ranked among the top 50 European orthopedic implant manufacturers by Research facts LTD in 2012
Biotechni products range , implant and associate instrument are CE 1639 certified
Quality Policy
BIOTECHNI designs and manufactures orthopedic implants distributed worldwide. Our Industry sector manufactures mechanical parts on behalf of third parties.
The total satisfaction of our customers in compliance with applicable regulatory requirements, the development of new product ranges to win new markets, and the continuous improvement of our processes are fundamental objectives.
To achieve these objectives, BIOTECHNI undertake to :
- Implement a quality management system meeting the requirements of EN ISO 9001 and EN ISO 13485.
- Comply with regulatory requirements applicable to medical devices and monitor those that could have an impact on the Industry sector.
- Adopt a process approach and a risk approach to promote the effectiveness of this Management system.
- Prioritize customer listening and ensure that at all levels, customer requirements are determined and respected.
- Communicate within the company the importance of meeting customer requirements as well as regulatory requirements.
- Support staff training to maintain competence at all levels.
- Provide a suitable organization, with formalized working methods and equipment controlled and maintained.
- Promote partnership relationships with our suppliers.
- Define quality objectives, compatible with the context and strategic direction, which will be reviewed periodically as part of the continuous improvement of our performance and the effectiveness of the quality management system.
- Communicate the necessary information regarding the quality management system to all levels of the company.

Production équipement
International Presence
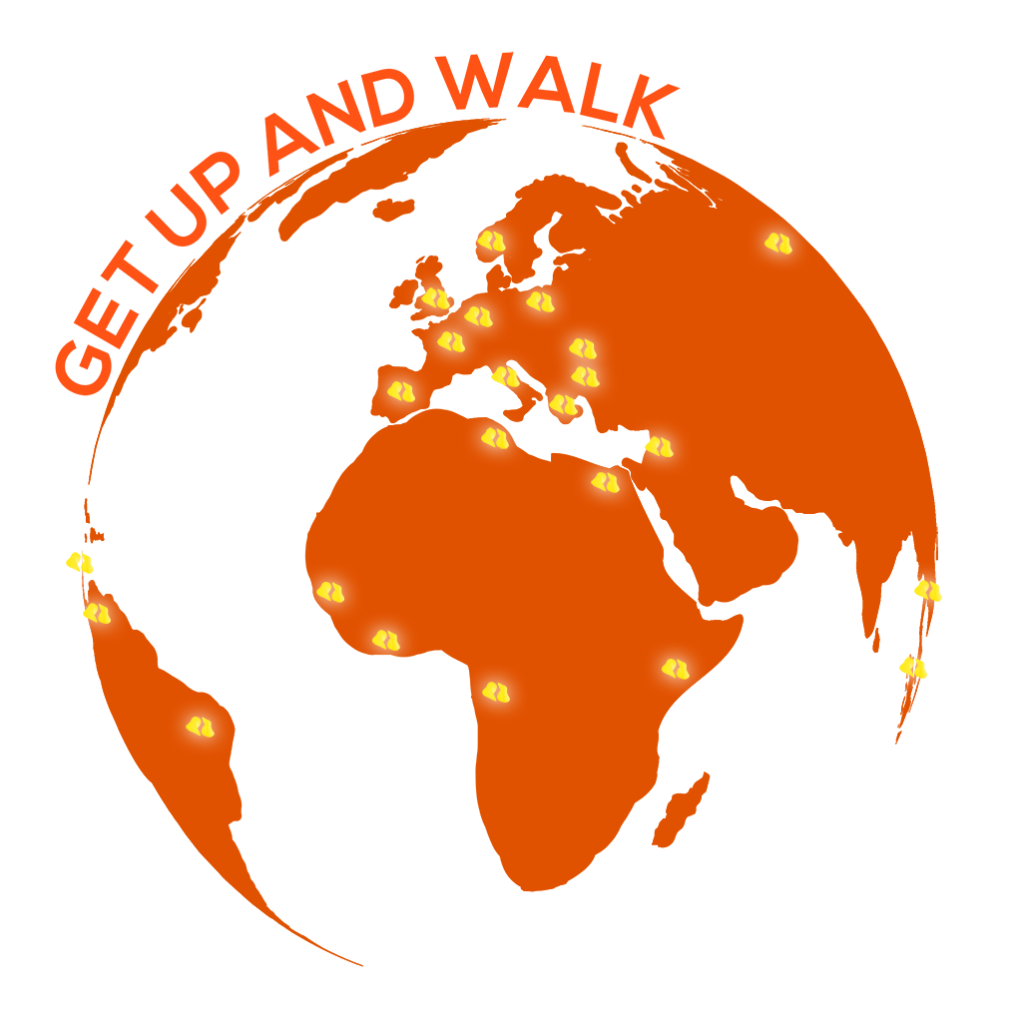
We are present in 27 countries
Taking advantage of its success with innovative implants on the French market, Biotechni has turned to the international scale in 1990.
Over the past three decades, Biotechni has been able to consolidate its positions on all continents. To date, our implants are sold in 27 countries, through an international network of trusted distributors.
Our flexibility and knowledge of the markets have seduced our international partners.
In the context of the economic and health crises facing the world, Biotechni is asserting its position and pursuing its commercial development.
France, Spain, Italia, Belgium, Norway, Bulgaria, Romania, Poland, Greece, Lebanon, UK, Russia, Egypt, Tunisia, Ivory coast, Senegal, Congo, Kenya, Vietnam, Indonesia, Japan, New Zeland, Brasil, Venezula, Colombia, Panama, Nicaragua.








